Last updated on August 20th, 2023 at 11:38 pm
Among the various fashion articles, denim is the fashion icon. For ages, denim has held its waist. Denim fabrics are made of different colors and different styles. Blue, black, or any other color is basically made with sulphur. In this article, we will discuss the critical points of sulphur dye.

Significance/Properties of Sulphur Dyes
- Sulphur Dyes are very cheap and available.
- This dye stuff is mostly used for cellulosic goods (i.e. cotton, jute etc.) colorization as because concentrated alkali is used there.
- Its tinctorial power is very low.
- This dye stuff is not soluble in water but we can solubilize with sodium sulphite as reducing agent in the presence of soda ash (Na2CO3).
- Its wet fastness properties is very good and light fastness properties is average.
- Some sulphur dye stuffs flooring fastness property is very weak.
Note: Tinctorial power of dye stuff is the intensity of color.
Chain formation of Sulphur Dye

Remarks/Precautions of Sulphur Dyes
- If any water contain calcium more or less as soon as sulphur color comes in contact with the water and produces calcium sulphate (S + H2O + Ca → CaSO4), Which make a few percent sulphur colors, to prevent it; the water should be totally free from calcium.
- Brass and cupper vessels are strictly prohibited in case of sulphur coloration. Only iron, wooden and earthen vessels are advised to use for sulphur coloration.
- In long storage of sulphur dyed material in open stage is very harmful as because it tender or damage the dyed fibric immediately. Chemical Reaction: S + H2O + O2 → H2SO4
Defects of Sulphur Dye
- There are two main defects found in sulphur dye:
- Broziness or dulness of shade
- Tendering or weaking.
Broziness of Shade
Causes:
- Use of excessive dye – stuff and salt.
- Use of inadequate Na2S.
- If the materials are not timely and perfectly washed after dyeing.
- If the materials are floated in the dye bath during boiling.
- Excessive heat.
Remedies:
- Treating with soap solution.
- Use very dilute Na2S bath.
- Boiling the materials in soap bath adding T. R. oil.
Tendering
Causes:
- If the dyed materials are stored, then this effect is seen. Here sulphur color is oxidized and produced H2SO4 which degrades cotton.
- After treating with cupper salt causes rapid tendering.
- Presence of iron as an impurity causes rapid tendering.
Remedies:
To remove that defect, the material is rinsed with K or Na – dichromate and then after treatment is done.
Application of Sulphur Dye on Cotton Goods
Recipe:
Sulphur color → 8% (According to the wt of tex. Mtls)
Soda Ash (Na2CO3) → 8%
Na2S (Reducing agent) → 8%
H2O → 20 times
Time → 1 Hour
Temperature → 90ᵒC – 100ᵒC
Dyeing Scheme:
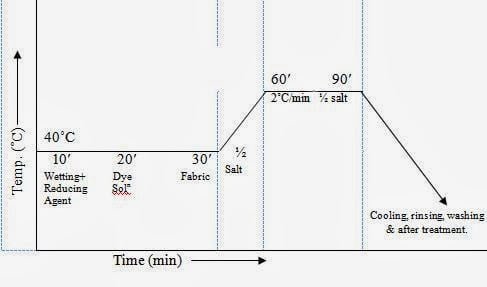
Working Procedure
To solubilize the dye stuff, first it is pasted with T.R. oil and wetting agent. Boiling water, Na2CO3 and Na2S is added and heated. This dye solution is taken to the dye bath and the fabric is given to the bath. Then 50% salt solution is given and then heated at boiling temperature. Rest 50% salt is given after 20 minutes. After 30 minutes dyeing at boiling temperature (100˚C), the fabric is rinsed and washed with water.
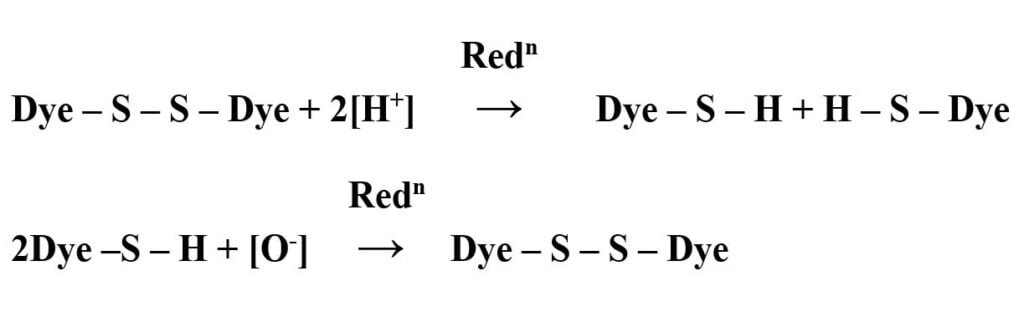
Oxidation:
After dyeing, the reduced water soluble form of the dye is oxidized to fix the dye stuff to its insoluble form. (1.5% K2Cr2O7 and acetic acid is used for oxidation. Total reaction:
After treatment:
The light of sulphur dye is generally good but it can be improved further more by a treatment with 2% of each CuSO4 and CH3COOH in a bath rising temperature up to 70˚C for 20 – 30 minutes.
Similarly, washing fastness can be slightly be improved by treating the material in a bath containing 2% each K2Cr2O7 and CH3COOH and rising temperature up to boiling point for 20 -30 minutes.
Recipe for after treatment:

Stripping
Stripping means removing uneven shade from the material. In case of sulphur dyed materials, warm solution of 8% Na2S is used in presence of 3% Albigin – A (Stripping promoter) for stripping.
In this method it is insufficient to remove the uneven shade. Then we have to use 3% NaOCl (Bleaching chemical) for over oxidation for destroying the dye take – up by the material. When the dye is completely oxidized and removed, then the material is suitable for topping.
Topping
To minimize the cost of Aniline Black and Indigo Blue, sulphur color may be topped with Aniline Black and Indigo Blue.
With basic color – sulphur dye acts as a mordant for basic dye and hence cotton materials dyed with sulphur color can be topped with basic dyes for brightening the shades. The combined dyeing however has the light fastness of basic dye. In this case, sulphur dyed material should be washed thoroughly to remove the alkali from the material. In the second dye bath which contain ½ – 1% basic color 2% – 3% CH3COOH or Alum and temperature should be raised to about 60˚C to control the exhaustion of the basic dye for 30 minutes.
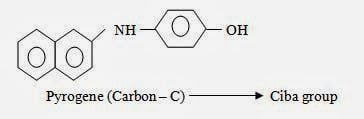
Chemical Structure of Sulphur Dye
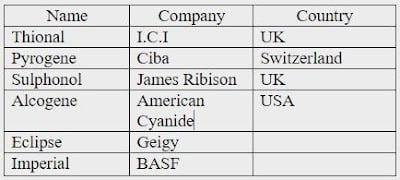
Commercial Name of Sulphur Dye
Test Method of Sulphur Dye
Boil sulphur dyed material in a test tube with 2% stannous chloride (SnCl2) and 2% HCl. At that time, if a piece of filter paper soaked in a solution of Pb – Acetate is placed on the mouth of the test tube, the paper will be turn into a black or brown.





Important topic…
Sulphur Black dyes manufacturer, please contact me at mark121928@gmail.com