Textile Bleaching [A to Z]
Last updated on October 7th, 2023 at 12:39 am
Definition of Textile Bleaching
Bleaching is the 3rd step of wet processing. The process by which the natural color of a fibre can be removed and make the textile material pure white and bright is called bleaching.

Objects of Bleaching
There are many objects as follows:
- To remove the natural color.
- To make the fabric permanent white and bright.
- To increase the absorbency power of the cloth.
- To increase dye affinity.
- To ensure level dyeing property.
- To make the fabric suitable for the next subsequent process.
Types of Textile Bleaching Agent
They are in two types as follows:
- Oxidizing and
- Reducing
Oxidizing Bleaching Agent
The chemical agents which remove coloring material from textile materials by oxidizing reaction and make the material pure white and bright and to increase absorbency power is called oxidizing bleaching agent. Ex: O3, Ca(OCl)Cl, H2O2, NaOCl, Ca(OCl)2, K2Cr2O7 etc.
Generally natural vegetable fibres are bleached by these bleaching agents.
Reducing Bleaching Agent
The chemicals which removes coloring materials from textile materials by reduction reaction and make the material pure white and bright and increase absorbency power is called reducing bleaching agent. Ex: H2S, FeSO4, NaHSO4, Na2SO4 etc.
Generally protein fibre i.e. silk, wool, etc. are bleached by these agents.
Factors/Conditions of Bleaching Process
Bleaching process depends on some important conditions. Some important conditions are given below:
- Concentration of the chemical.
- M : L ratio.
- PH of the liquor.
- Temperature of the liquor.
- Time of treatment.
- Catalyst.
Machine Chemicking Bleaching Process
Machine chemicking process is one of the discontinuous or, batching processes. This process takes more time.
Recipe:
HOCl: 3 – 4 Tw0
Temperature: Below 400C
Time: 8 – 12 hrs
PH: 9.2 – 11

Bleaching solution is to be prepared according to the above recipe. Here, material and liquor ratio is less. First the fabric is openly passed through the guide roller and immersed into the bleaching solution by immersion roller.
Then the fabric is squeezed by a pair of squeezing roller and pilling in a definite box. This fabric is kept in this box for a certain period for bleaching action. Then the fabric is treated with 0.5% HCl or H2SO4.
Faults in Bleaching and Their Remedies
Several faults of Bleaching are as follows:
- Incomplete and uneven bleaching
- Over bleaching
- Weaken the fabric.
- Roughness of the textile material.
- Yellowish effect over the textile material.
Incomplete and Uneven Bleaching
Causes:
- For using insufficient bleaching chemicals.
- Uncontrolled temperature.
Remedies:
- Sufficient chemicals should be used.
- Temperature should be controlled.
Over Bleaching
Causes:
- For using excess/over chemicals.
Remedies:
- Sufficient chemicals should be used according to the weight of the textile material.
Weaken the Fabric
Causes:
- Prolong stay in the sunlight causes weaken the fabric.
- Incorrect concentration of the solution.
Remedies:
- We should protect the bleaching process from the sunlight.
- Concentration of the solution should be correct.
Roughness of the Textile Materials
When we use calcium hypochlorite for bleaching purpose, hypochlorite is reacted with the atmospheric carbon-dioxide and thus produces calcium carbonate which is precipitated. Calcium carbonate is insoluble in water and if attached to the fabric surface that makes fabric rough.
Ca(OCl)Cl + CO2 → CaCO3↓ + Cl2
Remedy:
- Fabric should be scoured for removing calcium carbonate.
Points to be Considered Before Bleaching
The points that are considered are given below:
- In case of proteinous fibre reducing type of bleaching agent is used.
- If proper bleaching is not done, it will not give proper result.
- Jute, Hemp, Flax type fibres required two types of bleaching chemicals, Firstly, oxidative is used and finally the reducing type agent is used.
- Most of the bleachings are performed in the presence of ideal bleaching agent. H2O2 may be termed as ideal bleaching agent because, it is very cheap. It has no side effect.
Bleaching of Cotton by Hydrogen Per-Oxide (H2O2)
Thenard produced H2O2 from BaO2 and dilute HCl in 1818. He named this as oxygenated water.
BaO2 + 2HCl → BaCl2 + H2O2
Recipe:
H2O2 (35% S): 0.75 litre/kg (According to the wt. of the material)
NaOH: 0.05 kg
Na2CO3: 0.02 kg
Wetting agent: 1%
NaSiO2: 0.07 kg
H2O2 (50%): 0.5 l/kg
H2O: 100 l/kg
H2O2 is an oxidizing agent which is used largely for cellulose fibre bleaching. H2O2 is also used for protein fibre such as wool, silk-bleaching etc. In H2O2 bleaching, H2O2 releases hydrogen ion (H+) and per hydroxyl ion (HO2–). Here, PH level is maintained 10.7 – 10.9.
Per hydroxyl ion is released for alkalinity of hydrogen per-oxide solution. This per ion bleached the textile materials. H+ ion has no bleaching action. It maintains PH level.
(HO2–) + Colored materials = Bleached materials + H+
H2O2 is normally unstable, moreover Cu, Ni, Fe, etc. catalyst decomposes H2O2 and produces H2O and O2.
2H2O2 + Catalyst → 2H2O + O2
The produced O2 has no bleaching action. So, bleaching ability can be reduced if water contain Cu, Ni, Fe, etc ion, then H2O2 catalytically decomposes and reduces bleaching ability. So, all the above matters should be considered in H2O2 bleaching.
You may also like: Objects of Singeing | Gas Flame Singeing Machine

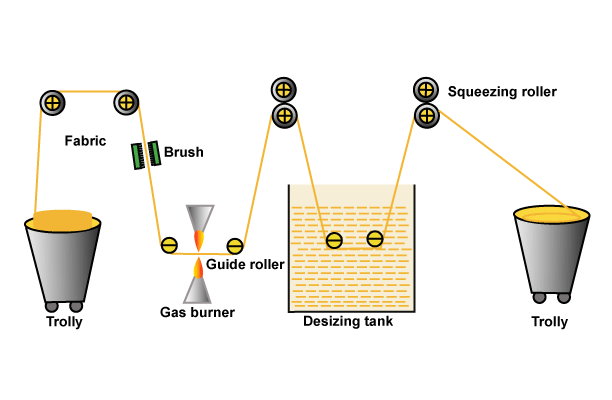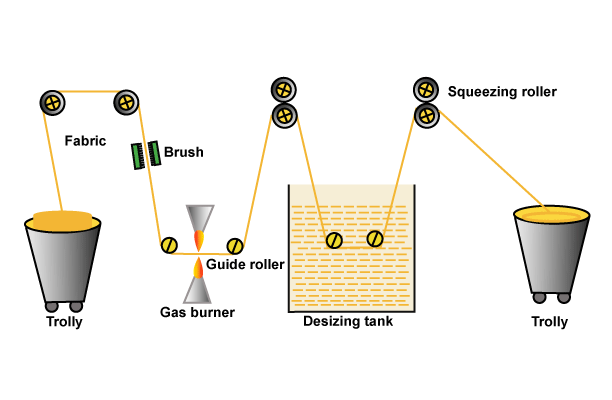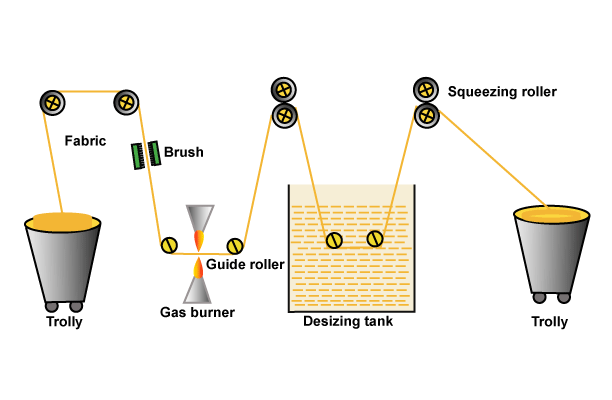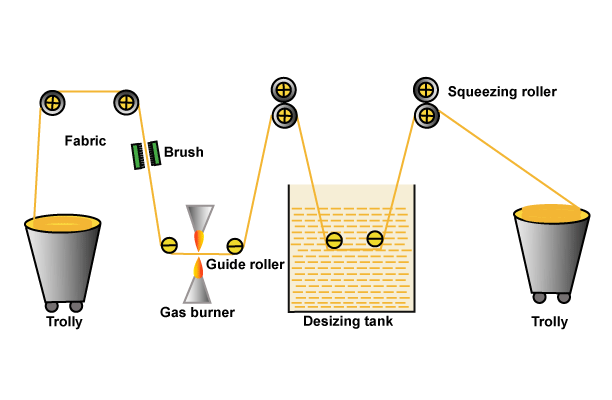

Would you explain the use of the flowering plant, lanaria, for finishing or bleaching textiles? Thank you