Hydrolysis of Reactive Dye
Last updated on August 23rd, 2023 at 11:46 am
Definition of Hydrolysis
Hydrolysis is a chemical process in which a molecule is cleaved into two parts by the addition of a molecule of water. One fragment of the parent molecule gains a hydrogen ion (H+) from the additional water molecule. The other group collects the remaining hydroxyl group (OH−).
Hydrolysis of Reactive Dye
During the application of reactive dyes to cellulose fibers under highly alkaline conditions, a competing hydrolysis reaction takes place, originating in the non-reactive oxi-dye form, which is lost for dyeing, and passes into the waste water.
Unfixed or hydrolyzed reactive dye has to be washed off thoroughly in order to achieve the desired superior wet fastness of the reactive dyeing. As much as 50% of the total cost of a reactive dyeing process must be attributed to the washing-off stages and treatment of the resulting effluent. This aspect of the process should be recognized as a major limitation that prevents reactive dyes from achieving the degree of success that was predicted for them at the time of their discovery.
In case of Triazinyl dyes:

In case of Vinyl Sulphone Dyes:
Dye-SO2CH = CH2 + HO – cellulose ® Dye – SO2 CH2 -CH2 O cellulose
Dye-SO2CH = CH2 + H-OH ® Dye-SO2 CH2-CH2 OH
For preventing hydrolysis the following precautions are taken:
- As hydrolysis increases with increasing temperature during dissolving and application temperature should not be more than 40°C.
- Dye and alkali solution are prepared separately and mixed just before using.
- Dye and alkali should not be kept for long time after mixing.
Exhaustion, Fixation & Hydrolysis
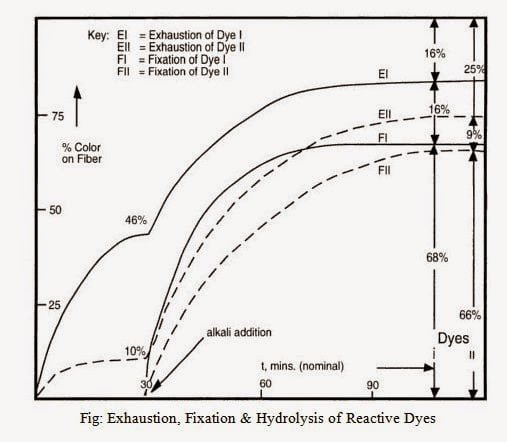
Here,
- The percentage of hydrolyzed dye exhausted onto the fibre: 16% & 9%
- The percentage of dyes I & II actually Fixed: 68% & 66%
- The percentage of hydrolyzed dye dropped with the dye bath: 16% & 25%
- Together (2+3) indicates the percentage of wasted color: 32% & 34%
Dye Hydrolysis In and Out the Fibre
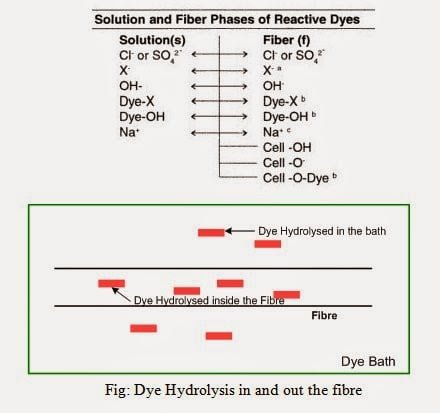
Factors Affect Dye Hydrolysis
- Liquor Ratio: Longer liquor ratio increases Hydrolysis and vice versa.
- Salt Concentration: Higher Salt Concentration decreases Hydrolysis upto a limit and vice versa.
- PH: Higher PH Increases Hydrolysis and vice versa.
- Temperature: Higher Temperature increases Hydrolysis and vice versa.
- Dye Reactivity: Increase both hydrolysis & fixation rate.
- Dye Substantivity: More substantive dye cause more hydrolysis but if substantivity is reduced to bare minimum or removed altogether, the build-up problem arises during dyeing and color yield problem (dyeing) of the dye is reduced considerably.
- Time: Higher Dyeing time increases Hydrolysis and vice versa.
- Type & number of Reactive Group: VinylSulphone is more prone to hydrolysis than Triazinyl group.
- Types of bridging group: The oxide (O-) and sulphide bridges are less stable in alkaline hydrolysis. But more stable bridges decreases reaction rate along with the hydrolysis.
Hydrolysis & no. of Reactive groups in a Reactive Dye
Covalent bond formation and hydrolysis take place concurrently during the dyeing of cellulose with a reactive dye. Clearly if the dye has only on reactive group, hydrolyzed product can no longer take part in the dyeing. However if the dye has more than one reactive group, it will contain further groups for opportunity for fixation.
Additional reactive groups do offer the important benefit of potentially increasing the fixation of a dye.
If the probability of fixation of each reactive group is, for example 60%, and if it is assumed that hydrolysis of the first reactive group does not alter the physical or chemical properties of the dye the probability of dye-fibre bond formation of a bifunctional dye is 84%[60+(0.6×40)]. Although all structural stages to a dye impact on its dyeing behavior some extent a multifunctional dye has a fixation potential than a nonfunctional reactive dye.
Potential Problem Due to Dye Hydrolysis
- Hydrolysis accompanies fixation, resulting in incomplete utilization of dye. Hence dye wastage occurs. Up to 40%-60% dyes (Avg. 50%) are wasted in this case.
- Relatively large amounts of electrolyte are required for exhaust, otherwise dye hydrolysis will occur greatly in dye bath.
- Laborious removal of unreacted and hydrolysed dye is required – often a longer operation than the dyeing step itself and not always entirely satisfactory.
- Longer washing operation for removal of unreacted and hydrolyzed dye often costs 50% of total dyeing cost.
- Hydrolyzed dye is discharged as coloured effluent, and effluent cost is risen up. Moreover, Color is not easily removed by effluent treatment processes and in many cases the dyes are not readily biodegradable.
- Hydrolyzed, unfixed halo heterocyclic reactive dyes may pose an environmental hazard (AOX).
- Hydrolyzed reactive dyes being covalently bound to the substrate, fastness problems associated can occur due to hydrolysis.
- Less Storage ability.
- Trailing Problem for continuous dyeing.
- Running Shade in batch process.
You may also like:




Very important article.
Only this post proved that any body is not equal you and your level
A complex subject illustrated simply. Thanks.
Hello I absolutely love your article and it has been so admirable hence I am going to save it. I Have to say the Superb research you have done is greatly
Hello! Good stuff, please keep us posted when you post again something like that!